Venn Diagram Between Ionic And Covalent Bonds

The other side has the bond compound type shop the black friday sale.
Venn diagram between ionic and covalent bonds. Ionic and covalent bonding is exothermic due to the fact that the energy of the participants is lowered. Ionic compounds are known as salts covalent bonds are a sharing of 2 electrons between the center of the two atoms being bonded covalent bonds occur between two non metals covalent bonds can be single double or triple. Get 50 off quizlet plus through monday learn more. Covalent bonds can be said when there is the strong electrostatic force of attractions between two positively charged nuclei and the shared pair of electrons.
Similarities in terms of electronic pair between ionic and covalent bonds. Key differences between covalent metallic and ionic bonds. Covalent bonding is a form of chemical bonding between two non metallic atoms which is characterized by the sharing of pairs of electrons between atoms and other covalent bonds. The similarities are both types of bonds.
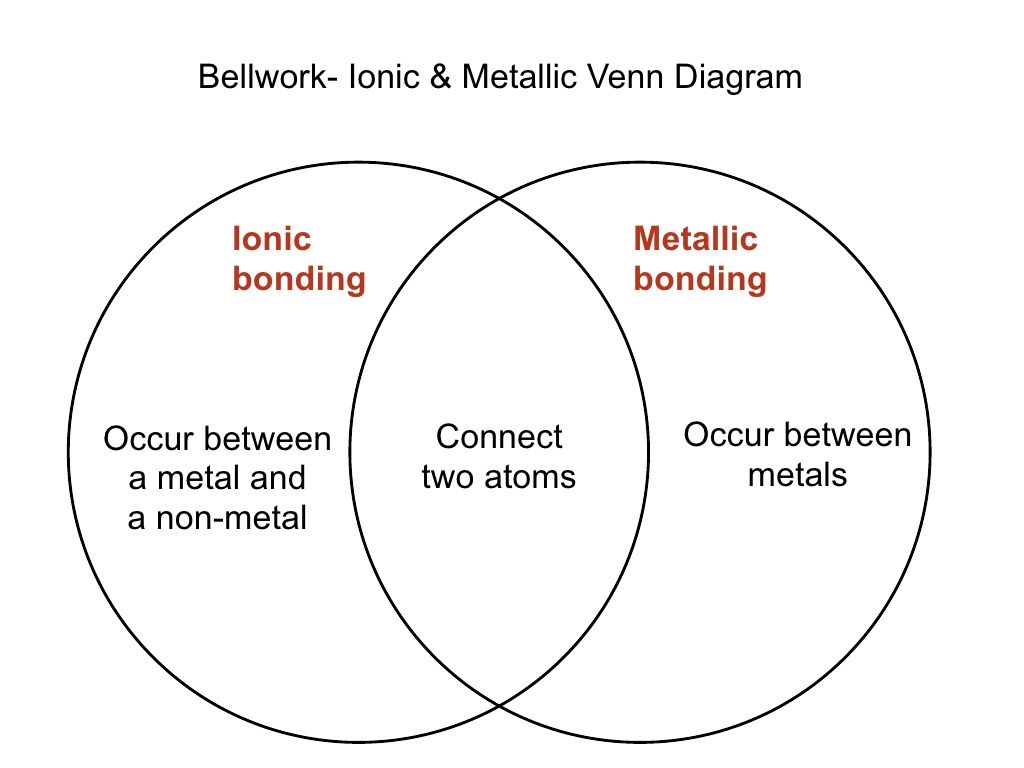
In nonpolar covalent bonds electrons are shared equally by both members of the bond but they are shared unequally in polar covalent bonds. Both types of bonding are carried out by a common electronic pair. Ionic bond also known as electrovalent bond is a type of bond formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Bellwork ionic metallic venn diagram ionic metallic bonding bonding occur between connect occur between a metal and two atoms metals a non metal 2.
Similarities in terms of electrostatic force between ionic and covalent bonds. Characteristics of ionic metallic and covalent bonds and compounds. Given below are the points which differentiate among the three types of strong or primary bonds. In a covalent bond the atoms are bound by shared electrons.
Ionic bonds are much stronger than van der waals forces which involve temporary sharing of electrons among molecules in a fashion more closely analogous to covalent bonding than to ionic bonding. Noble gases don t bond. Ionic bonds are between metal and non metal. Lecture 8 1 ionic vs.
Usually an electron is more attracted to one. Ionic is stronger bond than covalent. One side has the characteristic. In a true covalent bond the electronegativity values are the same e g h 2 o 3 although in practice the electronegativity values just need to be close if the electron is shared equally between the atoms forming a covalent bond then the bond is said to be nonpolar.